
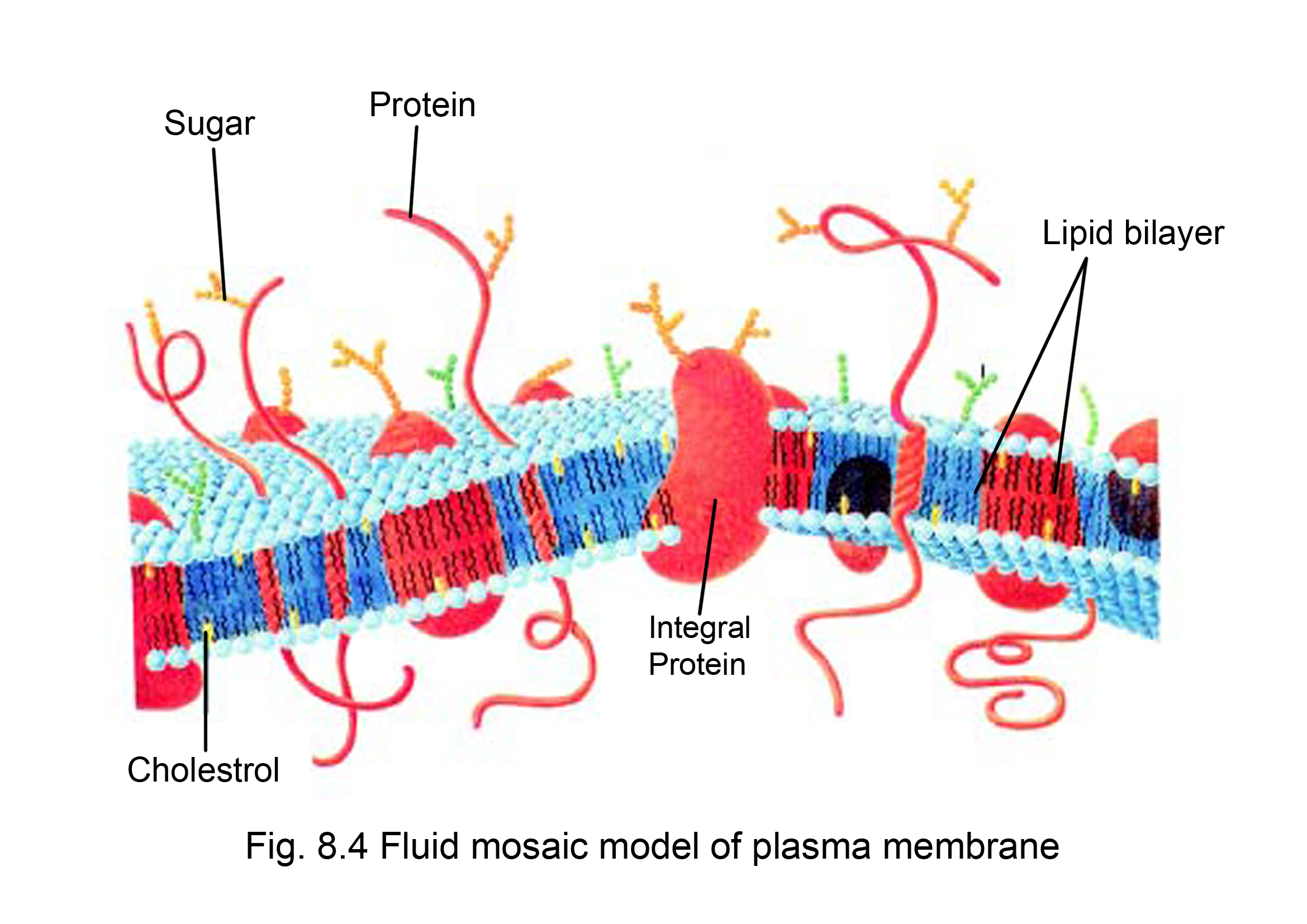
And then you have your hydrophobic tails, let me do that in a different color. This is the inner layer, and I'm doing a cross-section right over here. So that would be one layer of the phosphate heads facing the outside. So, I think I'll draw half of it, just because you get, well I'll draw the whole thing and hopefully you get the idea. So you could imagine something, let me see, if I drew a cross-section, let me see if I can draw it relatively neatly. You would think that if you go far back enough, even before life in cellular form, formed, that you might have had phospholipids spontaneously forming these spheres where you have a bilayer, a lipid bilayer. But the really cool thing is, a structure like this, having this Amphipathic molecule, allows things like these bilipid, these lipid bilayers, I should say, to form. And the tails are hydrophobic, the water's going to go away from them or they're going to go away from the water and so they're just going to face each other and they're going to be on the inside of the membrane. Because you could image, the hydrophilic heads are going to want to be where the water is, which is going to be either outside the cell or inside the cells. And hopefully that starts to explain why they organize themselves in this way. So phospholipids are Amphipathic, which means that they have both a hydrophilic end, a part that is attracted to water, and a hydrophobic end, that is not attracted to water. Amphipathic, a word that I sometimes have trouble saying. And actually molecules that have a hydrophilic part and a hydrophobic part, there's a special word for them. They're going to dissolve well, and so this part right over here, is going to be hydrophilic. Charged molecules do well in polar substances like water. And then you have the phosphate head right over here, and as you can clearly see, this has some charge. So you have hydrophobic tails, and these are really kind of the lipid part of the phospholipids. But these don't have those, and so they're not going to be attracted to the water and the water's not going to be attracted to it, to them, and so these tails are hydrophobic. We know that water's a polar molecule that's what gives it its hydrogen bonds, and it's attracted to itself. And that's true, as the case of this phospholipid, you have these hydrocarbon tails that are coming from fatty acids, and so these hydrocarbon tails, they have no obvious charge or no obvious polarity. And in general the word lipid, and we have a whole video on lipids, means something that doesn't dissolve so well in water. It's a lipid that involves a phosphate group. So this is indicative of a phospholipid and as its name implies, and let me write that down, this is a phospholipid. Because when you understand what a phospholipid is, it starts to make sense why it would form a bilayer like this, and why it's the basis for so many membranes in biological systems. And so when you hear that you might say, well, what is a phospholipid? And that's a good question. And when you zoom in, and this little part right over here, this is actually a phospholipid bilayer that forms it. If we look at it relative to this diagram, this is inside the cell, and this is outside. We're looking at a cross-section of its surface, Where down here, this is inside the cell. Now, why is it called the Fluid Mosaic Model? Well, if we were to look at a cell membrane and just to be clear what we're looking at, if this is a cell right over here, and this is its membrane, it's kind of what keeps the cell, the inside of the cell, separated from whatever is outside the cell. Let's explore the Fluid Mosaic Model of cell membranes.


 0 kommentar(er)
0 kommentar(er)
